|
|
Vol.
27 No. 5
September-October 2005
Polyaniline: Thin Films and Colloidal Dispersions (IUPAC Technical Report)
Jaroslav Stejskal and Irina Sapurina
Pure and Applied Chemistry
Vol. 77, No. 5, pp. 815–826 (2005)
Polyaniline (PANI) is one of the most important and widely studied conducting polymers. It is easily prepared (e.g., by the oxidation of aniline with ammonium peroxydisulfate in acidic aqueous medium) and obtained as a precipitate. Such synthesis has recently been investigated within IUPAC project 1999-024-1-400, “Polyaniline: Preparation of a Conducting Polymer,” and a report has been published in PAC 74, 857–867 (2002).
Polyaniline protonated with inorganic acids is difficult to process because it cannot be dissolved or melted below the decomposition temperature in the conducting state. The protonation of PANI with organic acids having a bulky hydrocarbon component has been used to increase the solubility of PANI in organic solvents and the plasticity. The uses of dodecylbenzenesulfonic acid, dinonylnaphthalenesulfonic acid, or diesters of sulfosuccinic acid may serve as examples. Various surfactants have also been used as a component of the reaction mixture for the same purpose. Alternative processing strategies consist in coating of the surfaces of various substrates with a conducting PANI film and in the preparation of PANI colloids. The latter forms, produced in situ during the polymerization of aniline, are discussed in this paper.
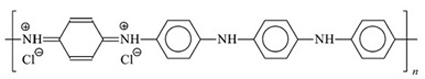 |
Polyaniline (emeraldine) hydrochloride (one of the possible presentations). |
An authority on conducting polymers has pointed out that “there are as many different types of PANI as there are people who synthesize it.” The purpose of this collaborative study was to test this statement, by having various researchers follow the same preparative protocol. Two supramolecular PANI forms, thin PANI films on glass and colloidal PANI dispersions stabilized with poly(N-vinylpyrrolidone) (PVP), were prepared independently in several laboratories. In this study, the films and colloids were characterized with respect to film thickness, film conductivity, and colloidal particle size.
The average thickness of the films, assessed by optical absorption, was 125 ± 9 nm, and the conductivity of films was 2.6 ± 0.7 S cm-1. Films prepared in 1 mol l-1 HCl had a similar thickness, 109 ± 10 nm, but a higher conductivity, 18.8 ± 7.1 S cm-1. Colloidal polyaniline particles stabilized with a water-soluble polymer, poly(N-vinylpyrrolidone) [poly(1-vinylpyrrolidin-2-one)], have been prepared by dispersion polymerization. The average particle size, 241 ± 50 nm, and polydispersity, 0.26 ± 0.12, have been determined by dynamic light scattering. The preparation of these two supramolecular polyaniline forms was found to be easily reproducible.
www.iupac.org/publications/pac/2005/7705/7705x0815.html
Page
last modified 22 August 2005.
Copyright © 2003-2005 International Union of Pure and
Applied Chemistry.
Questions regarding the website, please contact [email protected]
|