|
|
Vol.
28 No. 3
May-June 2006
How
to Access Structure and Dynamics of Solutions: The Capabilities
of Computational Methods (Special Topic Article)
Bernd M. Rode and Thomas S. Hofer
Pure and Applied Chemistry
Vol.
78, No. 3, pp. 525–539 (2006)
doi:10.1351/pac200678030525
Every experimental result is only as good as the theoretical
model employed for its interpretation. Usually there is a
complicated way from the actually measured data to the final
results; for example, the determination of a structure: A
theoretical model has to be defined, to which the measured
data are fitted until the "best possible" agreement
is achieved, mostly within a few percent of deviation. Though
not too error-prone in the case of highly regular solids,
this procedure becomes more difficult with gases (with their
high mobility of components) and with liquids (where high
mobility is combined with a density similar to solids). Any
a priori postulated models can be much too simplified.
 |
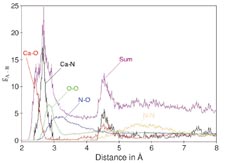 |
| One
of the ways in which simulations are superior to experiments
is that they offer the possibility of easily evaluating
any kind of atom–atom pair distribution. In more
complex systems (e.g., mixed solvents and solutions simultaneously
containing several solute species), this is an enormous
advantage over spectroscopic approaches, where only averaged
data (e.g., atom-atom distances) can be "seen."
The example shown in this figure illustrates the overlay
of various atom–atom radial distribution functions
for Ca(II) ion in aqueous ammonia [from A. Tongraar, K.
Sagarik, B.M. Rode. Phys.Chem. 4, 628 (2002)]. |
The
quality of theoretical models plays a pivotal role in the
determination of structural parameters, and even more, when
other physicochemical phenomena such as reaction dynamics
and mechanisms (where all interpretation of measurements depends
on a correct structural model plus corresponding mechanistic
models) are evaluated.
In
this article, the progress of computational chemistry in the
treatment of liquid systems is outlined. Emphasis is on the
combination of the statistical methods—Monte Carlo and
molecular dynamics—with quantum mechanics as the main
foundation of this progress. The difficulties of experimental
studies of liquid systems without having obtained sophisticated
theoretical models describing the structural entities and
the dynamical behavior of these liquids demonstrate that chemistry
research is in a transition phase, where theory and high-performance
computing have not only become a valuable supplement but an
essential and almost indispensable component to secure a correct
interpretation of measured data.
www.iupac.org/publications/pac/2006/7803/7803x0525.html
Page
last modified 25 April 2007.
Copyright © 2003-2007 International Union of Pure and
Applied Chemistry.
Questions regarding the website, please contact [email protected]
|