|
|
Vol.
30 No. 3
May-June 2008
Structure-Based Nomenclature for Cyclic Organic Macromolecules (IUPAC Recommendations 2008)
W. Mormann and K.-H. Hellwich
Pure and Applied Chemistry, 2008
Vol. 80, No. 2, pp. 201–232
doi:10.1351/pac200880020201
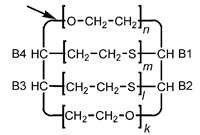 |
| Example 39 |
| Name: |
[B1],[B4]-
[poly(sulfanediylethylene)]- [B2],[B3]- [poly(sulfanediylethylene)]-
cyclo[poly(oxyethylene)- [1:B1][2:B2]ethylene- poly(oxyethylene)-
[1:B3][2:B4]ethylene] |
| or |
[B1],[B4]:[B2],[B3]-
bis[poly(sulfanediylethylene)]- cyclo[poly(oxyethylene)-
[1:B1][2:B2]ethylene- poly(oxyethylene)- [1:B3][2:B4]ethylene] |
A structure-based nomenclature system for monocyclic and polycyclic organic macromolecules is presented. Single-strand mono- and polycyclic macromolecules, as well as spiro macrocyclic compounds, are covered. However, rotaxanes and catenanes, which contain interlocked rings, and rings or ring systems formed by noncovalent bonds are excluded. Also, polypeptides and carbohydrate polymers are not included. The nomenclature of cyclic macromolecules is based on the existing nomenclature of regular and irregular macromolecules, which in turn is based on the nomenclature of organic chemistry, also published by IUPAC.
The procedure for naming a cyclic macromolecule consists of transforming it to an open-chain regular or irregular macromolecule in such a way that naming of units proceeds in descending order of seniority but otherwise follows the rules established for these types of macromolecules. For polycyclic macromolecules, the same principles are followed after the main ring, bridges, and branch units are identified and locants for branch units as well as bridges are assigned. The complete names are assembled by citing the component names and locants in the appropriate order according to the rules in this document. Wherever possible, examples for illustration of the naming procedure have been chosen from the literature.
www.iupac.org/publications/pac/80/2/0201
Page
last modified 5 June 2008.
Copyright © 2003-2008 International Union of Pure and
Applied Chemistry.
Questions regarding the website, please contact [email protected] |