|
News
& Notices
Organizations & People
Standing
Committees
Divisions
Projects
Reports
..By
Year
..Divisions
..Other Committees
..Provisional
Publications
Symposia
AMP
Links
of Interest
Search
the Site
Home
Page
|
Abbreviated list
of quantities, units and symbols in physical chemistry
6. Values of Some Fundamental
Constants#
| Physical Quantity |
Symbol for Unit |
Value in SI Units |
Unit |
| permeability of vacuum* |
µo |
4p x 10-7 |
N A-2 |
| speed of light in vacuum* |
co |
299 792 458 |
m s-1 |
| permittivity of vacuum |
eo |
8.854 187 816 x 10-12 |
F m-1 |
| elementary charge |
e |
1.602 177 33(49) x 10-19 |
C |
| Planck constant |
h |
6.626 0755(40) x 10-34 |
J s |
| Avogadro constant |
L, NA |
6.022 1367(36) x 1023 |
mol -1 |
| rest mass of electron |
me |
9.109 3897(54) x 10-31 |
kg |
| rest mass of proton |
mp |
1.672 6231(10) x 10-27 |
kg |
| Faraday constant |
F |
9.648 5309(29) x 104 |
C mol-1 |
| Hartree energy |
Eh |
4.359 7482(26) x 10-18 |
J |
| Bohr radius |
ao |
5.291 772 49(24) x 10-11 |
m |
| Bohr magneton |
µB |
9.274 0154(31) x 10-24 |
J T-1 |
| nuclear magneton |
µN |
5.050 7866(17) x 10-27 |
J T-1 |
| Rydberg constant |
R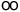 |
10 973 731.534(13) |
m-1 |
| gas constant |
R |
8.314 510(70) |
J K-1 mol-1 |
| Boltzmann constant |
k, kB |
1.380 658(12) x 10-23 |
J K-1 |
| gravitational constant |
G |
6.672 59(85) x 10-11 |
m3 kg-1 |
| standard acceleration due to gravity* |
gn |
9.806 65 |
m s-2 |
| triple point of water* |
Ttp(H20) |
273.16 |
K |
| zero of Celsius scale* |
T(0oC) |
273.15 |
K |
| molar volume of ideal gas (at 1 bar and
273.15 K) |
Vo |
22.711 08(19) |
L mol-1 |
#Cohen, E.R. and Taylor, B.N., J. Res. Nat.
Bur. Stand. 92 (1987) 85-95.
*These values are exact by definition
[Back to Index]
Page last modified 23 October 2003.
Copyright © 2000-2003 International Union of Pure and Applied Chemistry.
Questions or comments about IUPAC, please contact, the Secretariat.
Questions regarding the website, please contact web
manager.
|

