Ph.D. Thesis
Title Synthesis and Coordination Chemistry of
Chelating Verdazyl Radicals.
Adviser Dr. Robin G. Hicks
Thesis Committee Dr. David J. Berg, Department of Chemistry,
University of Victoria; Dr. Thomas M. Fyles, Department of Chemistry,
University of Victoria; Dr. J. T. Buckley, Department of Biochemistry
and Microbiology, University of Victoria; Dr. Robert C. Thompson,
Department of Chemistry, University of British Columbia.
Essay
Magnets serve an indispensable function in our technological-based
society and are ubiquitous in all varieties of mechanical and electronic
devices in science and industry. Traditional magnets are atom-based,
and are comprised of metals such as iron or nickel, the magnetism
arising from the magnetic dipole moment that is a product of the presence
of unpaired electrons. Recent synthetic efforts in chemistry have
focused on the preparation of molecule-based magnetic materials that
exhibit numerous desirable properties, including solubility, processibility,
and synthetic tunability—features that are a direct result of
their molecular nature and that are not shared by traditional atom-based
magnets. The magnetic properties of these new materials may also be
combined with other electronic or photonic characteristics of the
molecule, and it is expected that these new combinations will generate
novel properties. Such enthusiasm is embodied in the burgeoning fields
of spintronics and nanotechnology. Researchers studying magnetic materials
are very excited about the prospects for new nanoscale molecular materials
as functional magnetic memory devices leading to dramatically enhanced
data processing speeds and storage capacity in computers.
In order to rationally design a molecular magnetic material,
strong communication between atoms or molecules with unpaired electrons
(“spin”) is required. Specifically, the critical temperature
(Tc) below
which the solid exhibits bulk magnetic behaviour is directly proportional
to the strength (denoted by J, the exchange energy)
of local spin-spin interactions. The challenge for the synthetic chemist
is to chemically connect these spin-bearing building blocks
so that they will interact as strongly as possible. A particular subset
of building blocks that has been investigated in this regard is transition
metal coordination complexes containing stable free radical ligands.
This approach takes advantage of a strong direct magnetic interaction
between the unpaired spin(s) on the paramagnetic transition
metal centre, with the unpaired spin of the stable free radical ligand.
Using this metal-radical strategy, my research objectives
included investigating the magnetic interactions in simple coordination
complexes containing a radical of interest, and to develop structure-
property relationships from these investigations. However, the variety
of stable radical families is limited in scope. Extensive investigations
have been made with nitroxide or semiquinone radicals, but only modest
achievements have been obtained. There is much room for improvement,
and the current paradigm is that new designs of radicals are needed
in order to engineer enhanced new properties. Thus, my Ph.D. research
focused on a different family of stable free radicals as ligands—the
verdazyl radicals. No previous investigations had made use
of verdazyls as ligands until the present study was undertaken, despite
the system exhibiting a structure amenable to metal
coordination. Examples of selected verdazyl ligands that were prepared
over the course of this work are shown in Figure 1.
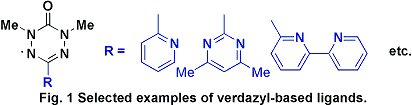
These molecules contain a variety of aromatic heterocyclic
substituents such as pyridine, bipyrimidine, and bipyridine, generating
bidentate, bisbidentate, and tridentate ligands,
respectively. Verdazyl radicals substituted in this manner are structural
mimics of the well-recognized family of oligopyridine ligands.
As an additional advantage, the rich and diverse history of transition
metal coordination chemistry exhibited by oligopyridine ligands augured
well for the potential coordinating
ability of verdazyl ligands.
In this research, using new synthetic protocols developed
in the investigation, nine such verdazyl derivatives were prepared,
and all were comprehensively characterized. These synthetic investigations
were followed by the preparation of over twenty crystalline metal-ion
complexes of this new family of ligands. An example of nickel(II)
or manganese(II) complexes containing a verdazyl ligand (containing
hexafluoroacetylacetonate ancillary ligands) is shown in Figure 2.
The complexes depicted in Figure 2 represented the first ever verdazyl
complexes with paramagnetic transition metal centers.
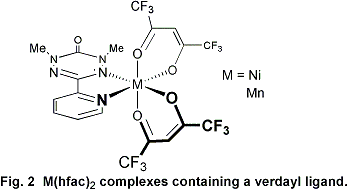
A series of spectroscopic studies together with molecular
structure determinations of the complexes and the parent radicals,
clearly demonstrated that judiciously substituted verdazyl radicals
were effective ligands and could bind to a wide variety of transition
metal centres. The molecular structures of three representative complexes
with the verdazyl ligands described in Figure 1 are depicted in Figure
3. Of note, verdazyl coordination complexes were, as expected, structurally
analogous to similar metal-oligopyridine complexes.

