Vol.
27 No. 1
January-February 2005
For more details on the chemistry involved, see the “Minireview” on conservation techniques by Heinz Langhals and Daniela Bathelt in Angew. Chem. Int. Ed. 2003, 42, pp. 5676–5681, and references therein.
Properties and Processing of Qi-Lacquer
Qi-lacquer is formulated from the sap of the lacquer tree (Rhus vernificera Stokes, new nomenclature: Toxicodendron vernicifluum [Stokes] F.A. Barkley) and is still used today. The lacquer is first conditioned by stirring and warming. The crude lacquer sap contains urushiol (1 in scheme on the right) and analogous components with a smaller number of double-bonds in the side chain. (The name urushio usually refers to a mixture of pyrocatechols with a side chain of 15 or 17 carbon atoms which contains up to three double bonds. The configuration at the double bonds and the exact composition depends on the source of the natural product mixture.) The content of urushiol should be more than 45% (preferably 70%) to obtain a smooth layer of lacquer. Hardening in air is induced by the copper-containing enzyme laccase (content 1% in the crude sap). Polymerization of urushiol proceeds through electron-transfer reactions with participation of both the aromatic structure and the side chains.
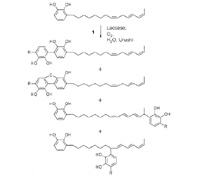 |
| Copyright 2003 Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim |
The formation of a smooth layer of lacquer is influenced by carbohydrate components, which give structure to the polymer. No nitrogen is detected in the hardened lacquer (detection limit inferior to 0.01 % by elemental analysis) as residues from laccase. A constant high humidity of 75–85% and a temperature of 25–30 ºC are important for the formation of a high-quality lacquer layer.
Page
last modified 13 December 2004.
Copyright © 2003-2004 International Union of Pure and
Applied Chemistry.
Questions regarding the website, please contact [email protected]
|