Chemistry International
Vol. 21, No. 6, November 1999
1999, Vol. 21
No. 6 (November)
..
40th Council Highlights
.. IUPAC: 2000 and Beyond
.. 37th IUPAC Congress
.. Chemistry in Today's Brazil
.. News from IUPAC:
Biodegradation of
Chemical Warfare
Agents
.. Other Societies
.. New Books and Publications
.. Provisional Recommendations
.. Awards
.. Conference Announcements
.. Conferences
Download the November
issue in pdf format.
(627K)
Download the November
cover in pdf format.
(117K)
Chemistry International
Vol. 21, No. 6
November 1999Biodegradation of Chemical Warfare Agents
Newer CW Biodegradation Research Efforts Show Progress
Several Approaches to Biodegrading Nerve Agents
Other Microorganisms with CW Hydrolytic Enzymes Identified
Strategies for Degrading Bulk Agents
Biodegrading the Blistering Agents HD and HT
Strategies for Degrading Organo-arsenical Blistering Agents
Unanswered CW Degradation Questions Require Further Research
Acknowledgments
Suggested ReadingThis article by Dr. Walter Mulbry ([email protected]), a microbiologist at the Soil Microbial Systems Laboratory, USDA/ARS, Beltsville, MD, USA, and Evgenia Rainina, a microbiologist at the Department of Biochemistry and Biophysics, Texas A&M University, College Station, TX, USA, constitutes the report of a Working Party of the IUPAC Ad Hoc Committee on Chemical Weapons Destruction Technologies (chaired by Professor Joseph F. Bunnett, [email protected]). It was originally published in ASM News, Vol. 64, No. 6, 1998, pp. 325-331, and it is reprinted here with the kind permission of the American Society for Microbiology. We thank the authors and Patrick Lacey ([email protected]), Production Editor at ASM News, for making it possible for us to publish this report.
The Chemical Weapons Convention (CWC), which the U.S. government ratified in 1997, sets an explicit timetable for signatory countries to destroy their chemical weapon stockpiles and related facilities. Although destroying such agents and remediating contaminated sites by conventional means promise to be enormously costly processes, recent research indicates that some of these compounds can be biologically degraded, suggesting an environmentally and economically feasible alternative strategy for addressing these challenges.
Figure 1. Artillery shells containing the nerve gas sarin (GB) inside a storage bunker at the Umatilla Chemical Depot in Oregon. (Photo courtesy of Donna Fuzi, Department of the Army)
The magnitude of the U.S. chemical weapon (CW) stockpileover 30 000 tons of various blister and nerve agentspresents a formidable challenge to those charged with disposing of it. About 60% of the agents are stored in steel 1-ton bulk containers; the remaining 40% are loaded in several million explosively configured rockets, land mines, mortars, bombs, artillery projectiles, and spray tanks (Figure 1). The "youngest" CW munitions and storage tanks are 30 years old, and the oldest are 53. In addition, U.S. Army officials have identified 215 sites in 33 states that are likely to contain buried chemical weapons or to be contaminated with chemical agents.
The financial resources needed for these tasks are equally formidable. Since 1985, the U.S. Army has spent USD 3.2 billion on its programs for destroying the U.S. CW stockpile and on planning for the treatment of material at nonstockpile sites (about 4% of these funds were used for research and development). The current cost estimate for the stockpile disposal program, USD 12.4 billion, has increased sevenfold since 1985 and is likely to increase further. In addition, U.S. Army officials estimate that at least another USD 16.6 billion will be needed over the next 40 years to treat buried material at nonstockpile sites.
Although few of the other CWC signatory nations have major CW stockpiles to deal with, several of the independent republics of the former Soviet Union, whose financial resources are already strapped, face cleanup tasks comparable to that of the United States. Other known CW concentrations include Japanese CW munitions abandoned in China in 1945 and an estimated 100 000 tons of German CW munitions that were dumped into the Baltic Sea at the end of World War II.
Newer CW Biodegradation Research Efforts Show Progress
For more than 50 years, the U.S. military and its counterparts in other countries have disposed of obsolete and surplus chemical weapons by a variety of means that are no longer acceptable. For instance, prior to 1969, the U.S. Army disposed of chemical weapons by open-pit burning, evaporative "atmospheric dilution," burial, and placement of munitions in concrete coffins for ocean dumping. In the 1970s, the U.S. Army began using alkaline hydrolysis to deactivate mustard agents known as H and HD (Figure 2).
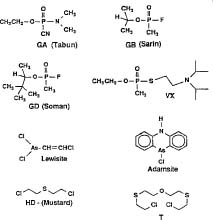
Figure 2. Chemical structures of the primary chemical warfare agents present in the U.S. and Russian stockpiles
Because of problems with GB alkaline hydrolysis, U.S. Army officials in 1982 adopted incineration for destroying all classes of chemical weapons. Subsequently, a full-scale CW incinerator facility was built in the Marshall Islands on Johnson Atoll and, more recently, a similar incinerator was built and brought into operation in Utah. Strong and effective opposition to incineration has, however, stalled U.S. Army plans to operate incinerators at seven other U.S. CW stockpiles. Similarly, strong opposition has stalled CW incineration projects in the independent republics of the former Soviet Union.
Historically, chemists and engineers who were involved in developing CW agents also were the specialists who studied how to dispose of these agents. However, in the late 1980s, the U.S. Army began a small intramural program on biodegradation at their Edgewood Research, Development and Engineering Center (ERDEC) in Aberdeen, MD. From 1991-1996, this program was broadened to support extramural research at several universities, including Texas A&M University, Rutgers University, and the University of Washington. In Russia, civilian research groups at Moscow State University, the Pushchino Biological Research Center, and several other government institutes have also been working on disposal strategies for agents in their stockpile.
These biodegradative research efforts are showing progress, particularly with efforts to dispose safely of both nerve and blister agents. Indeed, faced with strong political pressure to avoid incinerating such agents, U.S. Army officials recently changed CW destruction plans at one stockpile site, formally assigning biodegradation a role in treating HD stored in bulk containers.
Table 1. Bacterial strains with hydrolytic activities
against CW nerve agents
|
Hydrolytic activity [kcat (sec-1)] against: |
||||||
| Source Organism | Enzyme |
GA
|
GB
|
GD
|
DFPa
|
VX
|
| Pseudomonas diminuta | OPH |
NDb
|
56
|
5
|
465
|
0.3
|
| Alteromonas sp. JD6.5 | OPAA-2 |
94
|
611
|
3 145
|
1 650
|
0
|
| Alteromonas haloplanktis | NDb |
111
|
257
|
1 389
|
575
|
0
|
| Alteromonas undina | NDc |
300
|
376
|
2 496
|
1 239
|
0
|
|
a DFP, diisopropyl
fluorophosphate. A less toxic analog of the organophosphonate
agents. |
||||||
Several Approaches to Biodegrading Nerve Agents
The organophosphorus (OP) "nerve gases" include VX, GA (tabun), GB (sarin), and GD (soman), all of which are liquid with varied volatility at room temperature (Figure 2). The blistering agents are oily liquids at room temperature and include the "mustard" agents H, HD (agent H that has been purified by distillation), and HT (a 60/40 mixture of HD and agent T), as well as the organo-arsenical agent lewisite (L). Adamsite (DA), a second organo-arsenical agent, is a severe respiratory irritant. Mixtures of the mustard and arsenical agents were also produced, such as agent MLM (mustard-lewisite mixture, a 37/63 mixture of agents HD and L).
In the 1980s, investigators began to search for microorganisms that could metabolize CW agents or structurally related compounds. In the search for microorganisms to act on nerve agents, researchers focused on microbial isolates capable of hydrolyzing OP insecticides. Among a range of microbially derived OP insecticide hydrolyzing enzymes, only the enzyme OPH from Pseudomonas diminuta is at all active in degrading nerve agents (Table 1).
Recently, James Wild and his colleagues at Texas A&M University modified the cloned gene for OPH, changing the enzyme's catalytic specificity and increasing its ability to degrade two organophosphonate nerve agents. Thus, following site-directed mutagenesis, cells displayed a 4-fold increase in activity against VX and a 40-fold increase in activity against soman.
Collaborating researchers in other laboratories at Texas A&M University, the U.S. Army ERDEC, the University of Wisconsin, and the University of Pittsburgh are helping to define the catalytic and structural capabilities of the native and modified forms of OPH. For example, in 1994, Hazel Holden and her colleagues at the University of Wisconsin determined a crystal structure for this protein, which can be downloaded from the Brookhaven Protein Data Base on the World Wide Web at http://pdb.pdb.bnl.gov/ (PDB code 1PTA).
Other Microorganisms with CW Hydrolytic Enzymes Identified
In 1989, reasoning that microbial enzymes used in decontamination may need to be resistant to high salt concentrations, Joe DeFrank at the U.S. Army's ERDEC screened isolates from a hypersaline spring in Utah for CW agent hydrolytic activities. Among those isolates, he and his colleagues found several related Alteromonas isolates that efficiently hydrolyze nerve agents such as GB, GD, and GF (Table 1).
Subsequently, DeFrank and his colleagues Steve Harvey and Tu-chen Cheng at ERDEC characterized two related enzymes from these Alteromonas strains and isolated the gene (opaA) that encodes one of these enzymes (termed OPAA-2). The DNA sequence of opaA shares a 28% amino acid homology to Escherichia coli aminopeptidase P and human prolidase. Moreover, OPAA-2 is highly active in hydrolyzing two dipeptide substrates of prolidase, namely Leu-Pro and Ala-Pro, but is not active against tripeptide substrates of aminopeptidase P or the dipeptides Pro-Leu or Pro-Gly. Such evidence suggests that OPAA-2 is a prolidase having a role in peptide metabolism.
Although no further screening efforts have been undertaken, a more comprehensive screening of microbial dipeptidase activities from different sources may yield entirely new enzymes with higher activities or activities toward other nerve agents such as GA and VX.
Strategies for Degrading Bulk Agents
The Chemical Weapons Convention specifies not only that CW agents be destroyed irreversibly but also that particular by-products be destroyed to ensure that they cannot be reused for military purposes. The by-products also need to be rendered safe for discharge into open environments. Hence, the members of several research groups are studying whether biodegradation will serve to destroy the by-products remaining following either chemical or enzymatic hydrolysis of CW agents. This research has yielded promising results for treating both nerve and mustard agents.
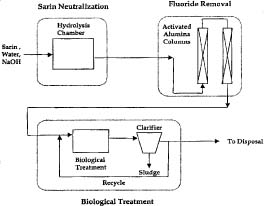
For example, Robin Autenrieth and her collaborators from Texas A&M University and the U.S. Army's ERDEC are studying the steps required to generate an environmentally acceptable waste stream following hydrolysis of sarin. She proposes hydrolyzing sarin with an excess of sodium hydroxide, a process that yields a solution containing sodium isopropyl methylphosphonate
(IMPA) and sodium fluoride, both of which must be processed before discharge (Figure 3).
Figure 3. Schematic of a proposed strategy for the treatment of sarin from bulk containers. In this process, sarin is chemically hydrolyzed using excess sodium hydroxide, fluoride is removed by absorption to alumina columns, and the resulting effluent is treated in bioreactors. (Figure courtesy of Robin Autenrieth, Texas A&M University)
Although attempts to use IMPA as a carbon source to support microbial growth were not successful, IMPA is metabolized as a sole source of phosphorus in bioreactors. In batch experiments where sarin hydrolysate is supplied to microorganisms growing in bioreactors, IMPA levels decrease from 85 mg/liter to their detection limit of 1 mg/liter within 60-75 hours, depending on the microbial consortia in use. However, the consortia require a six-week acclimation period, are inhibited by high levels of the sarin hydrolysate or other sources of phosphate, and require significant amounts of carbon and nitrogen to achieve the required C:N:P ratio of 100:12:1.
The structurally similar nerve agent soman can be treated by comparable hydrolytic and biodegradation steps. However, to reduce the acclimation periods and to raise growth rates when suspended cultures are supplied with soman hydrolysate, Evgenia Rainina at Texas A&M University tested whether immobilized microbial cells metabolize propylmethylphosphonic acid (PMPA) (the primary product of soman hydrolysis) as a sole phosphorus source.
For such experiments, a PMPA-degrading strain from the ERDEC collection is first grown to a high density in rich medium, immobilized within a poly(vinyl) alcohol cryogel, and incubated in phosphate-limited media for 24 hours to reduce endogenous phosphorus pools. In successive batch experiments using immobilized cells, PMPA levels decrease from 164 mg/liter to the detection limit of 1 mg/liter within 60 hours. These results demonstrate the potential utility of the cell immobilization technique for maintaining high enzymatic activities.
Applied separately, water or hydrogen peroxide can effectively hydrolyze another organophosphonate agent, VX, providing a promising first step along the path to degrading this compound. Whether water or hydrogen peroxide is used, ethylmethylphosphonic acid (EMPA) is a by-product that needs to be further degraded. It, too, can serve as a sole phosphorus source for natural microbial consortia in bioreactors.
However, the use of VX, soman, or sarin hydrolysates as phosphorus sources requires the addition of excess nitrogen and carbon, and is rate-limiting. Introducing cells containing constitutive C-P lyase activities into these consortia might accelerate the overall degradative process and thereby reduce the need for adding large amounts of nitrogen and carbon.
Biodegrading the Blistering Agents HD and HT
The poorly soluble blistering agent HD reacts with microbial proteins and is therefore highly toxic to microbial cells, making it a poor candidate for direct biodegradation. Alkaline hydrolysis of HD yields significant amounts of polymerized by-products that also are difficult to biodegrade, according to Yu-Chu Yang and colleagues at the U.S. Army's ERDEC. However, Steve Harvey and his colleagues at ERDEC recently determined that water at 90 oC effectively hydrolyzes HD, yielding 90-95% thiodiglycol (TDG), which is nontoxic and miscible in water.
Because the CWC mandates destruction of TDG, Harvey has been testing an aerobic sequencing batch reactor for its ability to biodegrade hydrolyzed HD products. Using activated municipal sludge as an inoculum and HD hydrolysate as a sole carbon source, this batch process yields a nontoxic effluent. Meanwhile, a cryoimmobilized culture of Alcaligenes xylosoxidans from ERDEC metabolizes 150 mM TDG within 24 hours and retains 100% of its initial activity after 4 months of continuous use, according to Evgenia Rainina.
Although the two-step strategy of hydrolysis followed by biodegradation readily applies to bulk containers of HD (purified mustard) and HT, its applicability to stockpiled U.S. munitions containing HD or agent H (unpurified mustard) is less certain. The stockpiles of other nations include the less expensive agent H rather than agent HD. Depending on how those materials were manufactured, agent H typically contains about 30% impurities and may also contain an array of thickeners and other additives. In many cases, during the decades of storage these materials have reacted with one another and with metal casing materials, forming solid or semisolid polymers that are recalcitrant to degradation.
(NEXT)
News
and Notices - Organizations and People
- Standing Committees
Divisions
- Projects - Reports
- Publications - Symposia
- AMP - Links
Page last modified 1 October 2001.
Copyright © 1999-2001 International Union of Pure and Applied Chemistry.
Questions or comments about IUPAC, please contact
the Secretariat.
Questions regarding the website, please contact [email protected]